Current Research on Molecular Processing
Radiation damage, a new
understanding
In the life sciences the role of electron-driven processes is being recognized as crucial to our understanding of radiation damage of cellular material. The mechanisms by which such degradation occurs have been the subject of considerable research effort with genotoxic effects of ionizing radiation in living cells being commonly attributed to direct impact of high-energy quanta or by complex radical chemistry (triggered by production of OH species by primary ionizing radiation). However recently this explanation has recently been questioned by the pioneering work of Sanche and co-workers who suggest that DNA lesions are induced by the lower energy, secondary electrons generated by the primary ionizing radiation. Data of Sanche and co-workers (figure3) revealed that :
- low energy electron irradiation directly induces both single and double strand breaks at energies well below the ionization limit of DNA (7.5eV) and
- that the probability of strand breaks are one to two orders of magnitude larger for electrons than for photons of corresponding energy.
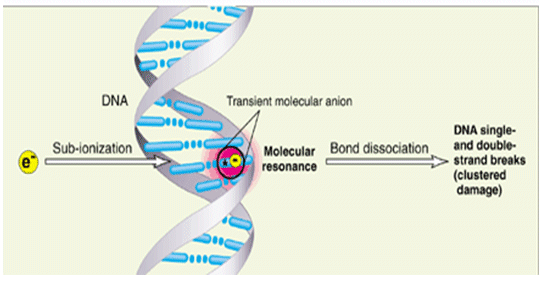
Figure 3: Most energy deposited in cells by ionizing radiation is channelled into free secondary electrons with energies between 1 eV and 20 eV (B. Boudaïffa et al., Science 287 (2000) 1658)
The majority of the copious secondary electrons (~5 x 104 per MeV) created within 10-15s along the radiation track have energies below 20 eV (Figure 4) . These low-energy electrons must undergo multiple inelastic scattering events as they thermalise. The primary energy-loss channels for electrons with energies typical of the secondary distribution are ionization, direct electronic excitation and most important (but until recently little studied) resonance scattering. The latter results in the formation of Temporary Negative Ions (TNIs), which decay via electron autodetachment and dissociative electron attachment (DEA) the latter process leading to direct dissociation of the parent molecule as noted above. This process may be summarised in the two step ‘reaction’;
e + M-ABC ---> (M-ABC) (1)
(M-ABC)- ---> M- ABC (2)
where (M-ABC)- is the temporary negative ion which decays to a residual anion (M-) and a (reactive) molecular fragment ABC, for example this may be the case in the STM experiment mentioned above where e + C6H5I->C6H5 +I-.
Hence the process of DEA provides a direct low energy process for the degradation of DNA (and other key cellular material) by resonant electron attachment to basic molecular components (base, deoxyribose, phosphate, or hydration H2O). Recent data suggest that single strand damage is site specific and proceeds through discrete molecular bond rupture. This in turn suggests that double strand breakage is simply induced through local chemical reactivity. The DEA fragments produced within the cellular DNA subsequently reacts with adjacent bases (at 3.4Å), or close-lying the phosphate-sugar backbone (at < 5Å) leading to clustered damage within the DNA strand.
Hence in contrast to traditional models of radiation chemistry which are based upon statistical modelling it would appear that cellular damage may be based upon single collisional phenomena involving transient molecular resonances localised on DNA basic components.
In this project we wish to explore this hypothesis through an intercomparison of the TNI formed from nucleotide bases and damage in DNA (and RNA) during low energy electron irradiation.
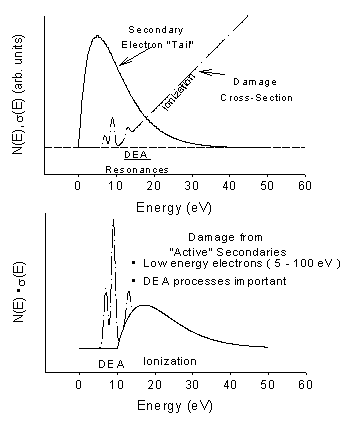
Figure 4: Top Frame: Typical energy distribution of secondary electrons emitted during a primary ionizing event. Also shown are the estimated relative cross-sections for some of the main inelastic electron energy-loss channels that the secondaries undergo as they traverse the interface. Lower Frame: Estimate of the effective “damage” probability obtained by convoluting the energy-loss cross sections with the secondary electron energy distribution.