Current Research on Molecular Processing
Nanotechnology and surface
engineering
Attempts to control chemical processes at the fundamental level using electronic excitations are now possible by combining the Scanning Tunnelling Microscope (STM) with an understanding of dynamics of electron-molecule interactions. For example, one of the most exciting developments in the area of surface modification is the use of STM to cleave specific bonds on surfaces and adsorbates. Many of these unimolecular reactions are mediated by vibrational coupling of electronic energy into vibrational motion, placing energy into specific reaction coordinates. These “molecular surgery” techniques allow control of local phenomena introducing the prospect of designer synthesis on the nano-scale.
As an exciting example of achieving the goal of controlled chemical synthesis consider one of the simplest and most well-studied reactions, oxidation of CO to CO2, an archetypal reaction in heterogeneous catalysis and an elementary reaction that is central to automobile emissions control, air purification, and chemical sensing. Using a STM Ho and Hahn [Phys. Rev. Lett., 87, 166102 (2001)] gently coaxed a CO molecule towards a pair of closely spaced oxygen atoms on a 10K silver surface. At such low temperatures molecular vibrations are minimized confining reactants and intermediates to specific sites on the surface. As the CO molecule is moved to within 2 Ĺ of the oxygen atoms, the atoms and molecule form an O-CO-O complex. Then, by applying a brief electron pulse from the STM the complex is excited to form CO2, which desorbs from the surface, leaving behind a lone oxygen atom (figure 1).
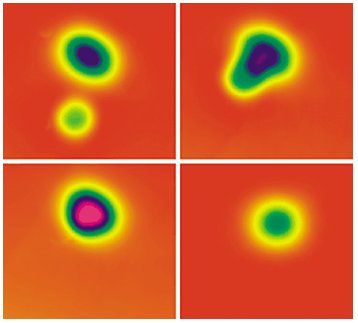
Figure 1. A pair of oxygen atoms (oblong) and a CO molecule (circular) appear as distinct features in an STM image (top left). As the species are brought closer together (top right), the image changes until, at less than 2 Ĺ separation, an O–CO–O complex forms (bottom left). Exciting the complex causes CO2 to form and desorb, leaving behind a lone O atom (bottom right).
Chemical reactions may also be induced by low lying transient anion states formed by the attachment of electrons to the parent molecule. Such quasi-bound states form since molecules are very ready to accept electrons into vacant molecular orbitals. In the case of dissociative electron attachment (DEA) in many systems a 100 % selectivity with respect to the cleavage of a particular bond can be obtained with a high cross section. This opens interesting prospects for selective chemistry induced by electrons. For example thermal electrons with CCl4 react to yield exclusively Cl- and CCl3. The reactive (radical) CCl3 may then be transported by the STM tip to another part of the surface where it may react at a specified chemical site. Such Single Molecule STM Chemistry has already been demonstrated for the iodobenzene molecule C6H5I (figure 2)
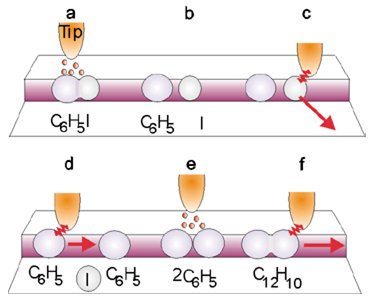
Figure 2: Schematic drawing of the sequence of steps by which an STM probe can (a) dissociate a C6H5I molecule on a terrace; (b and c) draw the iodine atom away; (d) pull one C6H5 (phenyl) molecule toward another; (e) weld them together; (f) pull one phenyl to confirm the association. Hla et al, Physical Review Letters 85 2777 (2000).
Thus our understanding of the interaction and manipulation of molecules by electron processing is of key importance to the development of nano-scale processing. This in turn requires closer co-operation (co-ordination) of research in fundamental atomic, molecular physics and surface sciences communities